|
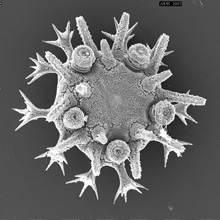
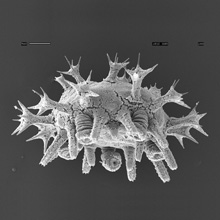
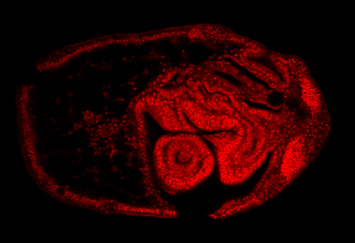
Minsuk, S. B., F. R. Turner, M. E. Andrews and R. A. Raff (2009). Axial patterning of the pentaradial adult echinoderm body plan. Development Genes and Evolution 219:89-101. [journal link] [download pdf] *
Minsuk, S. B. and R. A. Raff (2005). Co-option of an oral-aboral patterning mechanism to control left-right differentiation: the direct-developing sea urchin Heliocidaris erythrogramma is sinistralized, not ventralized, by NiCl2. Evolution & Development 7:289-300. [journal link] [download pdf] *
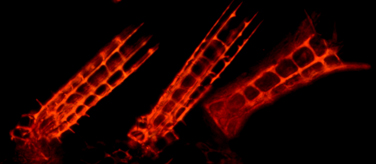
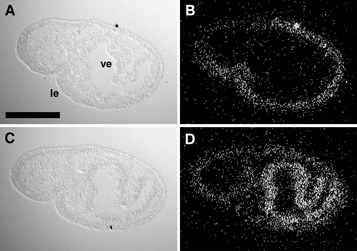
Minsuk, S. B., M. E. Andrews, and R. A. Raff (2005). From larval bodies to adult body plans: patterning the development of the presumptive adult ectoderm in the sea urchin larva. Development Genes and Evolution 215:383-392. [journal link] [download pdf] *
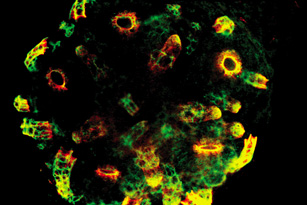
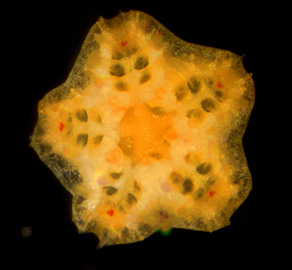
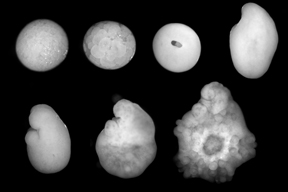
Minsuk, S. B. and R. A. Raff (2002). Pattern formation in a pentameral animal: induction of early adult rudiment development in sea urchins. Developmental Biology 247:335-350. [journal link] [download pdf]
Minsuk, S. B. and R. E. Keller (1997). Surface mesoderm in Xenopus: a revision of the stage 10 fate map. Development Genes and Evolution 207:389-401. [journal link] [download pdf] *
Minsuk, S. B. and R. E. Keller (1996). Dorsal mesoderm has a dual origin and forms by a novel mechanism in Hymenochirus, a relative of Xenopus. Developmental Biology 174:92-103. [journal link] [download pdf]
* I originally provided only journal links for these, because I do not legally have the rights to my own papers, which are available from the journals for a fee. After the death of Aaron Swartz, I decided to make these papers available here. See:
New York Times
slate.com
#pdftribute
pdftribute.net
|